Improving PROTAC properties via single-point changes to linkers
We explore how computational methods can be applied to proteolysis targeting chimera (PROTAC) design, to effectively tackle some of the ...
News
sparkV10 is a powerful tool for generating bioisosteres and evaluating their biological similarity to the starting molecule. sparkV10 presents a large range of possible changes to a given molecule, each of which introduces a different set of properties.
Moreover, at the end of a project, a team can assess the bioisosteres that are presented by sparkV10 in the light of their experiences and before writing their patent applications, ensuring that the maximum coverage of intellectual property has been obtained in their claim definitions.
Sumatriptan was the first marketed triptan drug and is a mixed 5-HT1B and 5HT1D agonist used in the treatment of migraine. Sumatriptan has three clear portions to the molecule, the 5-substuent, the central indole and the amino side chain. In applying sparkV10 to Sumatriptan we show the versatility of the approach by running sparkV10 experiments on each of the three portions of the molecule:
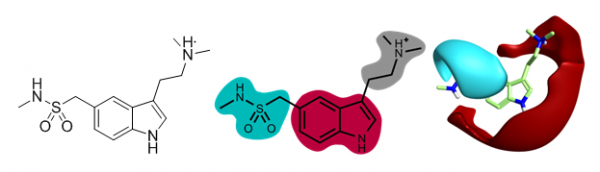
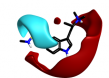
Selected results from each of these experiments are set out in the results table, below, together with the 3D field pattern for each result, the 2D similarity of the result to Sumatriptan, and the closest known active compound.
Bioisosteres for the Methyl Sulphonamide-Methyl Group
Search 1 provided an interesting mixture of known active compounds, analogues of known actives and unreported structures. Of note is the presence of numerous bioisosteres for the N-methylsulfamoyl-methyl group that most medicinal chemists would consider as obvious replacements. This is typified in Table 1 by the retroamide found at cluster 1-11, an analogue of Zolmatriptan XX and the methylsufonamido-ethyl group found at cluster 1-35.
However, the results also contain numerous 5- membered hetrocycles that could be considered to be less obvious bioisosteres of the sulfonamide, typified by the oxadiazole at cluster 7 and triazole at cluster 1-13.
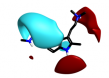
Some of these replacements are well known in the literature such as Rizatriptan [1], which was found in this search but somewhat lower down the result (at cluster 1-451). Most interestingly, search 1 also provided groups to be incorporated into Sumatriptan that could impart both activity and novelty such as the pyridone found at cluster 1-137. Examination of the 3D structure and electrostatic field for all these suggestions shows a highly conserved pattern that is remarkably similar to that obtained from the Sumatriptan query (Figure 1c).
Bioisosteres for the Central Indole
Search 2 was aimed at finding novel replacements for the central indole. Unlike the result from search 1 and search 3, there are few reports of rational changes that can be made to the indole such that activity is retained. Once again the results provided a mixture of obvious, conservative replacements and less obvious, more ambitious ideas.
Simple changes to the indole such as the introduction of an exocyclic atom (cluster 2-1) were common results. However, this modest change in a similar series reduces the potency against 5HT1B by a factor of 30 [2] suggesting a highly sterically confined binding site.
More radical changes such as reversing the indole (cluster 2-11) or shrinking the ring (cluster 2-8) or moving the indole NH to an exocyclic position (cluster 2-16) appear promising but are unreported and may also be victims of the sterically restricted binding site.
Interestingly there are reports of 5HT1B receptor ligands that lack the indole core [3] and incorporate a two ring structure such as in cluster 2-40 albeit in a non-aromatic framework that would adopt a significantly different shape to that shown in these results.
Bioisosteres for the Dimethylaminoethyl Group
Search 3 provided a host of amine containing replacements for the dimethylaminoethyl side chain of Sumatriptan.
The majority of the top scoring suggestions from sparkV10 have been observed in literature 5HT1B ligands such as the simple 2-methyl analogue 3-5 [4] or the pyrrole 3-16 [5]. Despite this, even in this context sparkV10 presents results that are not known in the literature such as the piperidine 3-31 and the cyclohexylamine 3-72.
Conclusions
The real power of this sparkV10 experiment is in presenting to a medicinal chemist a large range of possible changes to their molecule, each of which introduces a different set of properties and moves the drug discovery team in a different direction with respect to the multi-optimization problem they are facing.
Moreover, at the end of a project, a team can assess the bioisosteres that are presented by sparkV10 in the light of their experiences and before writing their patent applications, ensuring that the maximum coverage of intellectual property has been obtained in their claim definitions.
Results Table
| Search-Cluster Number | Result | Structure and Field | 2D SIM | Closest Literature | Ref |
| 1-7 |  |
 |
0.374 |  Found at cluster 451 |
1 |
| 1-11 | 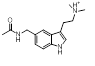 |
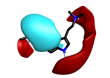 |
0.549 | 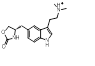 |
6 |
| 1-13 | 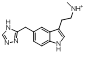 |
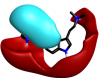 |
0.461 | 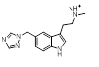 |
|
| 1-35 | 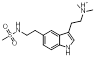 |
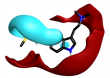 |
0.515 | n/a | |
| 1-137 | 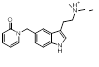 |
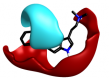 |
0.358 | 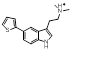 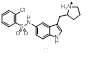 |
7, 8 |
| 2-1 | 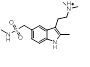 |
 |
0.762 | 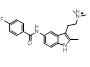 |
2 |
| 2-8 | 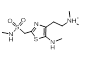 |
 |
0.247 | n/a | |
| 2-11 |  |
 |
0.501 | n/a | |
| 2-16 |  |
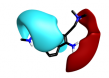 |
0.562 | n/a | |
| 2-40 | 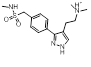 |
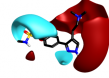 |
0.436 | 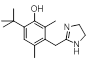 |
3 |
| 3-5 | 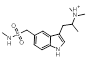 |
 |
0.754 | 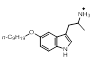 |
4 |
| 3-11 | 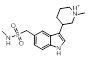 |
 |
0.631 | n/a | |
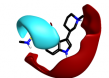
| 3-16 | 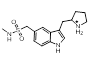 |
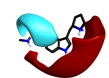 |
0.470 | 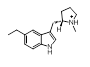 |
5 |
| 3-72 | 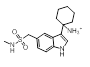 |
 |
0.431 | n/a |
References
1. Street, L. J.; Baker, R.; Davey, W. B.; Guiblin, A. R.; Jelley, R. A.; Reeve, A. J.; Routledge, H.; Sternfeld, F.; Watt, A. P.; Beer, M. S. Synthesis and serotonergic activity of N,N-dimethyl-2-[5-(1,2,4-triazol-1-ylmethyl)-1H-indol-3-yl]ethylamine and analogues: potent agonists for 5-HT1D receptors. J. Med. Chem. 1995, No. 38, 1799-1810.
2. Xu, Y. C.; Johnson, K. W. .; Phebus, L. A.; Cohen, M.; Nelson, D. L.; Schenck, K.; Walker, C. D.; Fritz, J. E.; Kaldor, S. W.; LeTourneau, M. E.; Murff, R. E.; Zgombick, J. M.; Calligaro, D. O.; J.E., A.; Schaus, J. M. N-[3-(2-Dimethylaminoethyl)-2-methyl-1H- indol-5-yl]-4-fluorobenzamide: a potent, selective, and orally active 5-HT(1F) receptor agonist potentially useful for migraine therapy. J. Med. Chem. 2001, 44, 4031-4034.
3. Law, H.; Dukat, M.; Teitler, M.; Lee, D. H.; Mazzocco, L.; Kamboj, R.; Rampersad, V.; Prisinzano, T.; Glennon, R. A. Benzylimidazolines as h5-HT1B/1D Serotonin Receptor Ligands: A Structure-Affinity Investigation. J. Med. Chem. 1998, 41, 2243-2251.
4. Glennon, R. A.; Hong, S. S.; Bondarev, M.; Law, H.; Dukat, M.; Rakhi, S.; Power, P.; Fan, E.; Kinneau, D.; Kamboj, R.; Teitler, M.; Herrick-Davis, K.; Smith, C. Binding of O-alkyl derivatives of serotonin at human 5-HT1D beta receptors. J. Med. Chem. 1996, 39, 314-316.
5. Slassi, A.; Edwards, L.; O’Brien, A.; Meng, C. Q.; Xin, T.; Seto, C.; Lee, D. K.; MacLean, N.; Hynd, D.; Chen, C.; Wang, H.; Kamboj, R.; Rakhit, S. 5-Alkyltryptamine derivatives as highly selective and potent 5-HT1D receptor agonists. Bioorg. Med. Chem. Lett. 2000, 10, 1707-1709.
6. Perez, M; Pauwels, PJ; Fourrier, C; Chopin, P; Valentin, JP; John, GW; Marien, M; Halazy, S Dimerization of sumatriptan as an efficient way to design a potent, centrally and orally active 5-HT1B agonist. Bioorg Med. Chem. Lett. 1999, 8, 675-680.
7. Meng, C. Q.; Rakhit, S.; Lee, D. K.; Kamboj, R.; McCallum, K. L.; Mazzocco, L.; Dyne, K.; Slassi, A. 5-Thienyltryptamine derivatives as serotonin 5-HT1B/1D receptor agonists: potential treatments for migraine. Bioorg. Med. Chem. Lett. 2000, 10, 903-905.
8. Cole, D. C.; Lennox, W. J.; Lombardi, S.; Ellingboe, J. W.; Bernotas, R. C.; Tawa, G. J.; H., M.; Smith, D. L.; Zhang, G.; Coupet, J.; Schechter, L. E. Discovery of 5-arylsulfonamido-3-(pyrrolidin-2-ylmethyl)-1H-indole derivatives as potent, selective 5-HT6 receptor agononists. J. Med. Chem. 2005, 48, 353-356.