Future-proofing Cybersecurity in Drug Discovery
The pharmaceutical and biotech sectors suffer more data security breaches than any other industry, with 53% resulting from malicious activity. To protect against potential ...
News
Senexis scientists had found a series of peptides with promising biological activity. Cresset’s software was used to identify drug-like chemotypes to mimic the activity of these peptides. The work resulted in novel lead molecules for Senexis.
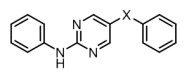
Senexis Ltd is a small molecule drug discovery company that has discovered novel compounds to treat diseases resulting from the toxicity of amyloid-like proteins. They had developed a series of ‘meptides’ (N-methylated peptides) that block the aggregation of β-amyloid. An example ‘L-meptide’ search molecule is shown in figure 1, below.
Senexis asked Cresset to find novel series of non-peptide small molecules that mimics the activity of the ‘meptides’. Cresset’s molecular field technology is ideal for such a purpose.
Cresset’s patented set of XED molecular mechanics algorithms describe molecules based on their molecular field, rather than chemical structure. The resulting field point descriptors of molecules give a powerful basis for analyzing and comparing molecules based on their activity.
Once a molecule has been defined according to its field pattern, it is possible to look for compounds with new chemistry that have the same activity, despite being from a different structural class.
The first step was to use torchV10 to find the best conformation for four of the meptides. A β-sheet conformation was assumed, the molecules were aligned and the field pattern was calculated for each meptide.
The aligned meptides were used as field seeds in Blaze (the new name for FieldScreen), Cresset’s ligand based virtual screening tool. Blaze compared the meptide’s fields to Cresset’s field database of 1M commercially available molecules. Cresset scientists reviewed and analyzed the hits.
Matches containing interesting and novel chemistry were reported back to Senexis for their review. Three of the interesting new chemotypes whose fields matched the input seed field are shown in figure 2, below.
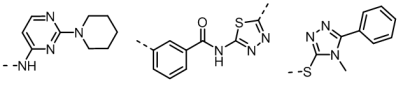
A further field seed from a pyridazine structure derived from RS0406, a known small molecule inhibitor, was then incorporated into the study. Results from a Blaze field search with this seed reinforced confidence in the validity of the new chemotypes.
Following this study, Senexis embarked on a program of medicinal chemistry and biological testing that resulted in two distinct chemotype sets. Cresset used Forge (the new name for FieldTemplater and FieldAlign) to produce templates for four active structures (two from each set) to find the common field pattern across all of the conformations and from that deduced the bioactive conformation.
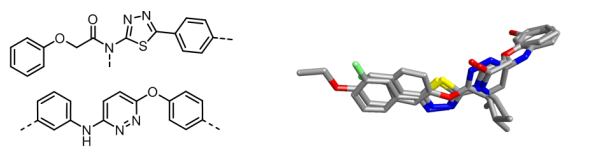
Further searches using these more reliable field patterns from the bioactive conformations revealed more information and ideas for the Senexis chemists to work with. Their current lead molecules are SEN1269 and SEN1186, the core of which is shown below.