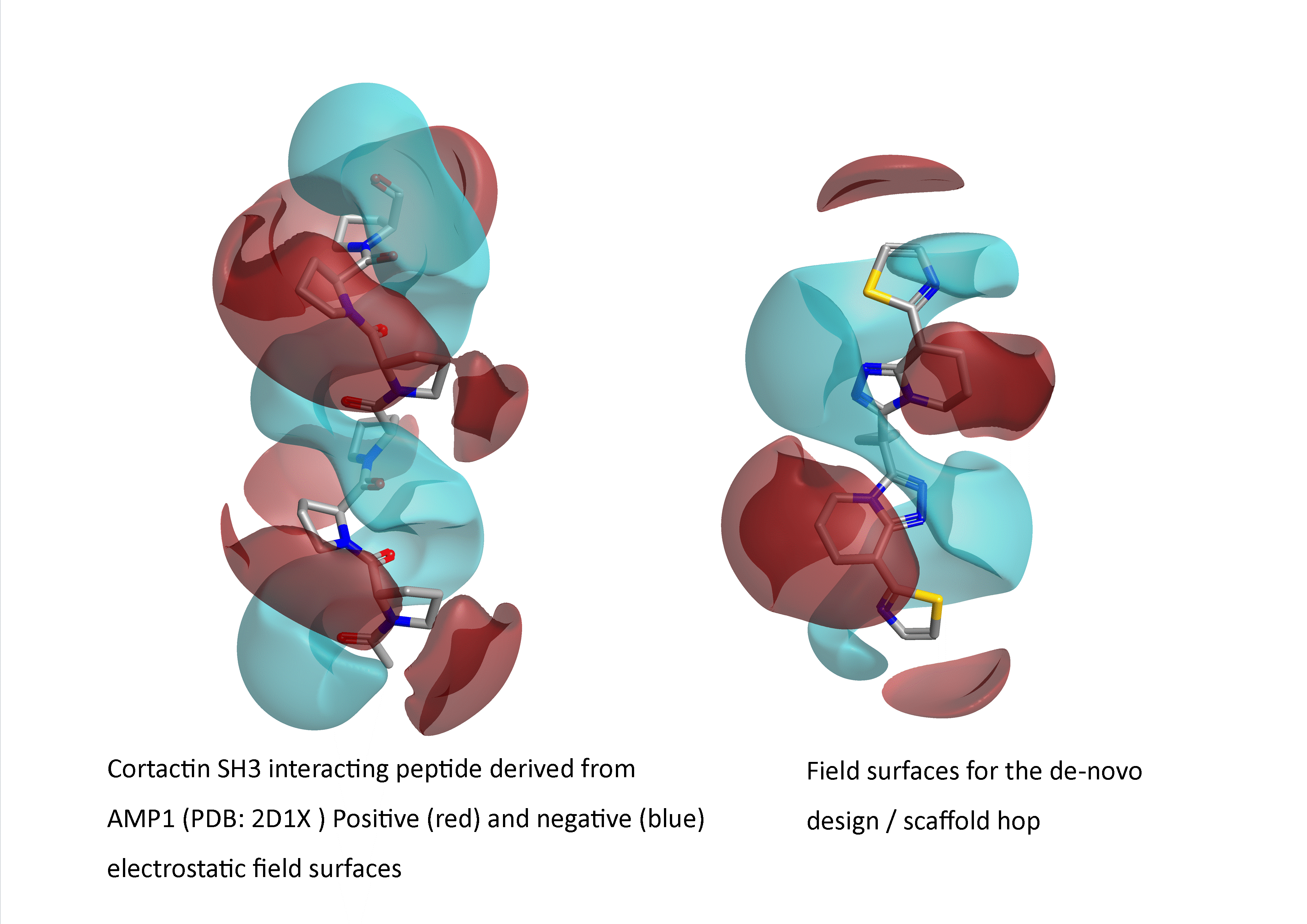One of the most popular requests for our consulting services team is to conduct ‘scaffold hopping’. The aim of scaffold hopping is to find new chemical structures with similar biological activity to the original by changing components of the molecule.
One issue with scaffold hopping is the phrase itself; it can mean slightly different things to different clients and can entail quite different scenarios. In drug discovery or a more general context, scaffold is usually defined as: ‘The central molecular component from which a specific combination of substituents or decorations define the interesting compounds within the series’.
It is this component that is the focus of the desired replacement or ‘hop’. The central component’s shape, electrostatic properties and vectors generally underlie the biological activity of interest, but it is the framework and combinations of decoration, defined in 2D, that hold the intellectual property.
Because the definition for protecting composition of matter under patent law relies on 2D structure and not biological activity, it is possible to take out a new patent on a structurally different molecule, despite it doing essentially the same job as an existing patented molecule. This sustains the so called ‘fast-follower’, ‘me-too’ and ‘me-better’ niche in the broader chemicals industry.
What are the strengths of virtual scaffold hopping?
The arguments for attempting ‘virtual’ scaffold hopping are similar to those already described for virtual screening, namely:
- Scaffold hopping is an effective way of identifying new intellectual property
- Virtual scaffold hopping is cheaper and quicker than wet scaffold hopping
- Using computational chemistry to investigate your lead series often leads to other insights into the mechanisms of biological action.
Scaffold hopping projects take a number of different forms, but generally engage the same technology used for virtual screening, making use of Cresset’s Blaze™ software. In this case the scaffold hop is achieved by using Blaze to search commercial compound vendor collections for a suitable ‘whole molecule’ replacement. This by definition produces new scaffolds as the output.
A second powerful software capability that we hold at Cresset allows us to explore changing one component of the active molecule at a time. This method is called ‘fragment replacement’ and is carried out using Spark. Rather than searching for commercial compounds to purchase, Spark provides ideas for new molecules that can be synthesized.
At Cresset’s recent North American user group meeting the independent medicinal chemistry consultant Alfred Ajami gave a presentation describing the use of Spark to retrospectively analyze two case studies from the bioisosteric replacement literature and to evaluate its performance in a number of projects that he has been involved with. For more information, see: Drug hunts with Spark: case studies from the literature and current campaigns to develop immunokinase inhibitors, Alfred M Ajami, DCAM Pharma Inc.
In the hands of trained users such as our experienced consulting team, both of these applications provide useful ideas for exploring new potential actives in a highly efficient manner.
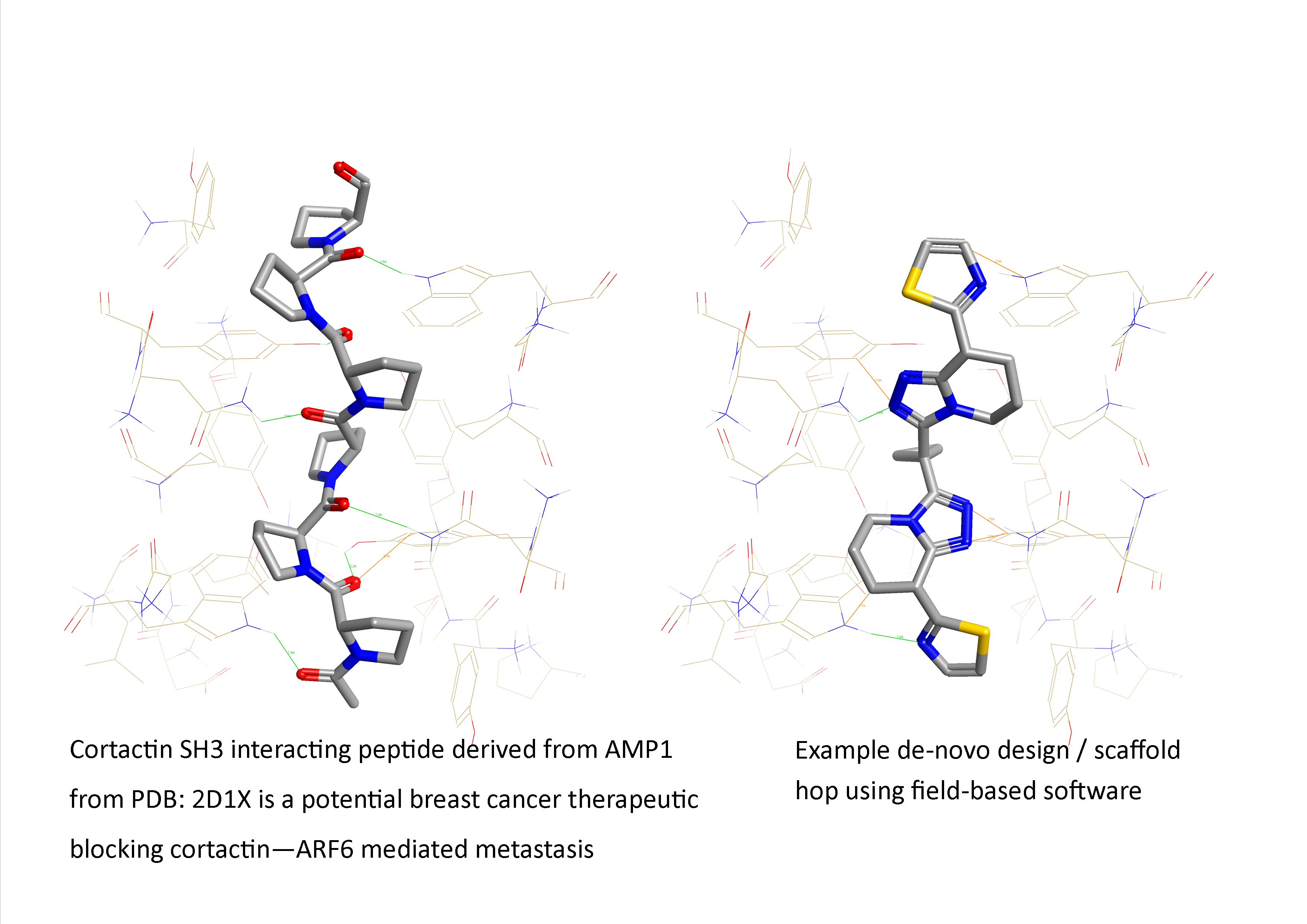
Figure 1: Example of a field-based scaffold hop from a therapeutically interesting peptide ‘AMP1 analogue to a small non-peptide synthetic mimetic (from PDB: 2D1X: an SH3 interacting peptide active in breast cancer metastasis inhibition).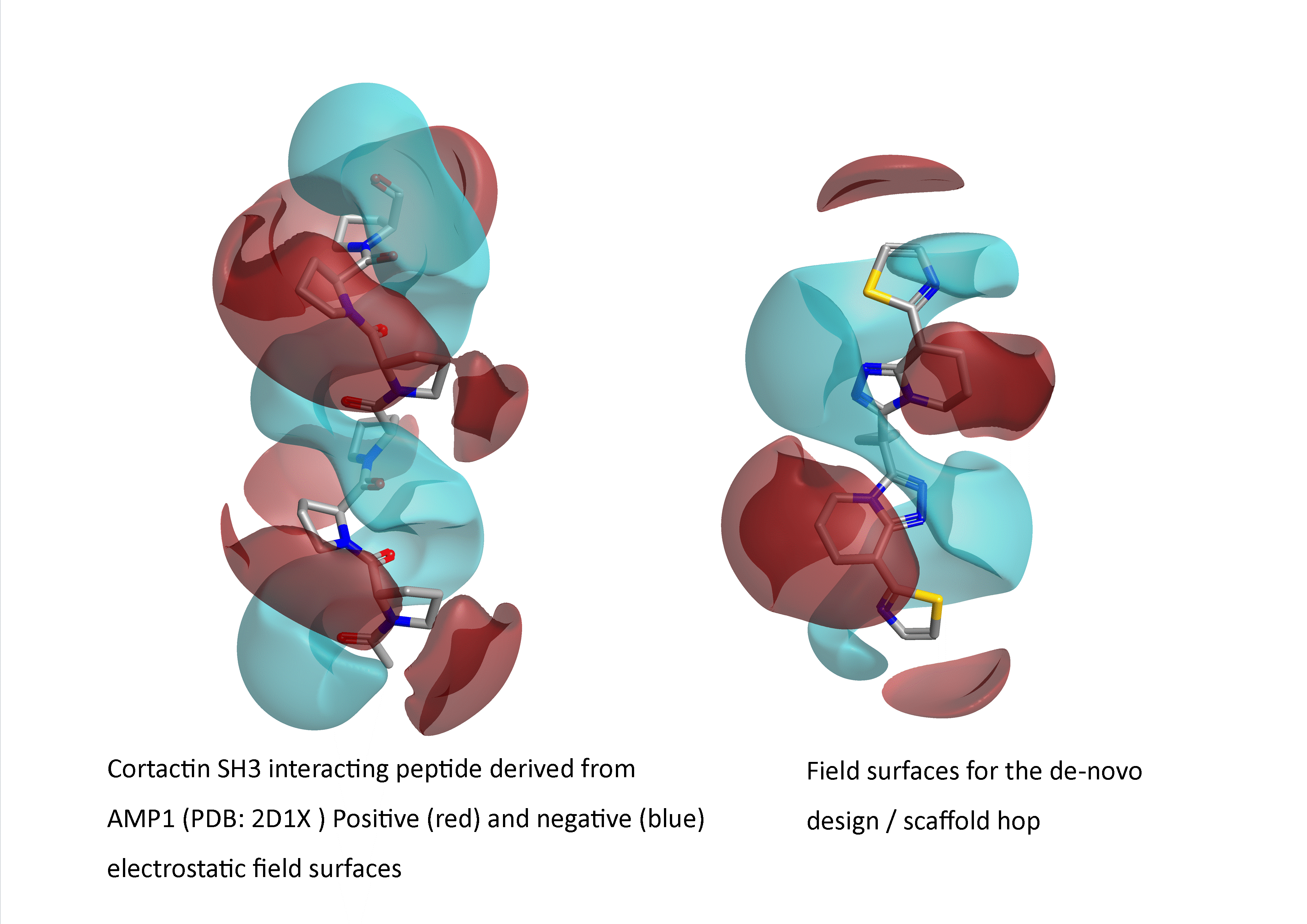
Figure 2: Comparison of the electrostatic field surfaces of the target and scaffold hop from Figure 1.
When should I employ this technique?
Scaffold hopping for replacement of a central heterocyclic core is a very common workflow. In spring 2013, Tim Cheeseright, Cresset’s Director of Products, presented a detailed study on the use of Spark for scaffold hopping from the core of Sildenafil. He showed that using Spark gave comparable results to those published by Pfizer but in a fraction of the time.
Scaffold hopping is a useful technique during the discovery phase of a project when there are no starting points other than a complex natural product. We have successfully used the scaffold hopping technique during several client projects to find small molecule actives with more tractable chemistry.
In the lead optimization phase where a current chemotype has proven active against the target biological system but has an intractable liability, both virtual fragment replacement protocols and whole molecule replacement are relevant.
One clear liability for an oral drug would be if the molecule is a protein, a small peptide or a nucleotide. Because the Cresset software is not dependent on the molecular framework, the output can be a small synthetic molecule derived from whatever input type was chosen, be that protein, nucleotide or cofactor. Cresset has performed several scaffold hops from an active peptide or cofactor.
Thus it can be seen that under the term ‘scaffold hopping’ we cover a far wider scope than the usual definitions of ‘scaffold’.
The following table summarizes the main areas in which scaffold hopping can be usefully deployed for drug discovery:
| Application Area |
Cresset Software |
| Hit to lead (fast follower) |
Blaze |
| Lead optimization (known liability avoidance) |
Blaze, Spark |
| Back-up series identification |
Blaze, Spark |
| Complex natural product to small molecule active |
Blaze, Spark |
| Protein – protein interaction inhibitors |
Blaze |
| Nucleotide – protein interaction inhibitors |
Blaze |
| Substrate-cofactor conjugate enzyme inhibitor |
Blaze, Spark |
Contact us for more information about how Cresset can apply scaffold hopping to your project.

Dr Martin Slater,
Director of Consulting