Future-proofing Cybersecurity in Drug Discovery
The pharmaceutical and biotech sectors suffer more data security breaches than any other industry, with 53% resulting from malicious activity. To protect against potential ...
News
Here at Cresset we live and breathe molecular fields – they are the core foundation of our technology. We use them for molecular alignment (Forge), for QSAR (Forge), for virtual screening (Blaze), for bioisosteres (Spark), and many other things besides. However, as well as using molecular fields to do nifty things, sometimes it can be extremely useful just to sit down and look at them. It’s something that within Cresset we tend to take for granted, but just viewing the molecular electrostatic potentials can give you all sorts of insights into the chemistry and properties of your molecules that are otherwise hard to get a handle on.
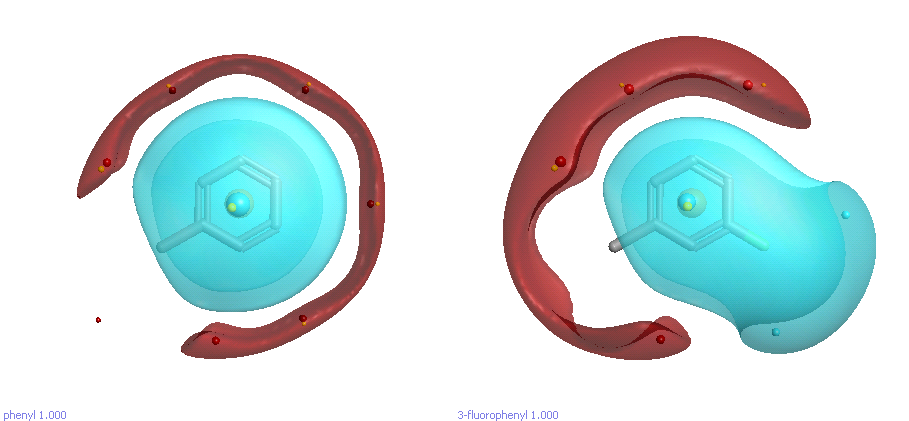
As a simple example, let’s suppose we’ve changed a phenyl to a 3-fluorophenyl substituent and got an activity increase (Figure 1). What’s the effect of the fluorine? Well, it’s pretty obvious that the fluorine is delta-negative, so there will be a region of negative electrostatic potential around it. So, the increased activity might be due to the fluorine interacting with a positive region of the active site. On the other hand, there’s a less-obvious effect of the fluorine: it polarizes the phenyl ring and makes the opposite site significantly more delta-positive. Perhaps the increase in activity isn’t directly due to the fluorine interacting with the protein; maybe it’s the other side of the phenyl doing the work (pi-pi stacking against a Phe or Trp, for example). That’s not obvious from the 2D structure, but is immediately apparent when you look at the field surfaces.
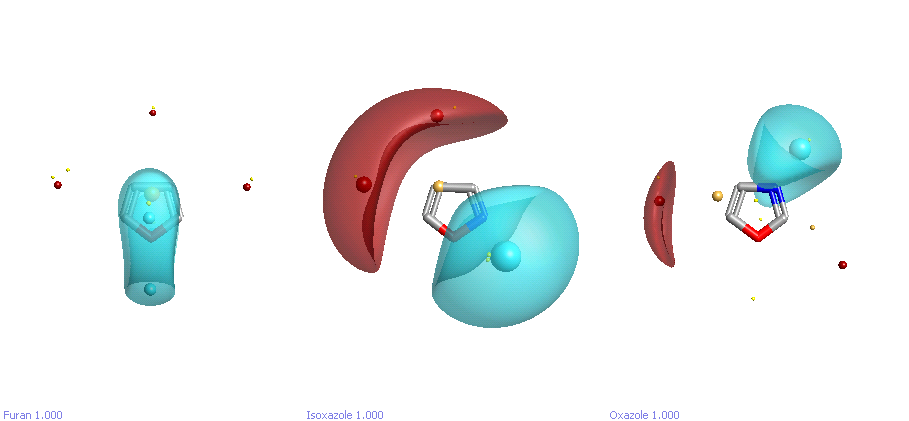
The field analysis really comes into its own when looking at heterocycles. For example, let’s compare furan, oxazole, and isoxazole (Figure 2). How are they going to behave in a protein binding context? Looking at the fields, you can immediately see the difference. Furan is mostly hydrophobic – there is some negative field potential off the oxygen, but it’s small (no larger than the potentials above and below the plane of the ring in benzene). By contrast, oxazole and isoxazole show strong H-bonding capability, in both cases off the nitrogen. It’s worth noting that the oxygen in oxazole shows virtually no negative electrostatic potential at all! If you look at the experimental interaction data from Isostar, you can see that NH and OH groups have an overwhelming preference to interact with the N rather than the O, confirming this. The last thing that becomes apparent from the electrostatic potential maps is the very large dipole of isoxazole compared to oxazole: these two ring systems are far from being bioisosteric, and so large differences in activity between these as substituents should come as no surprise.
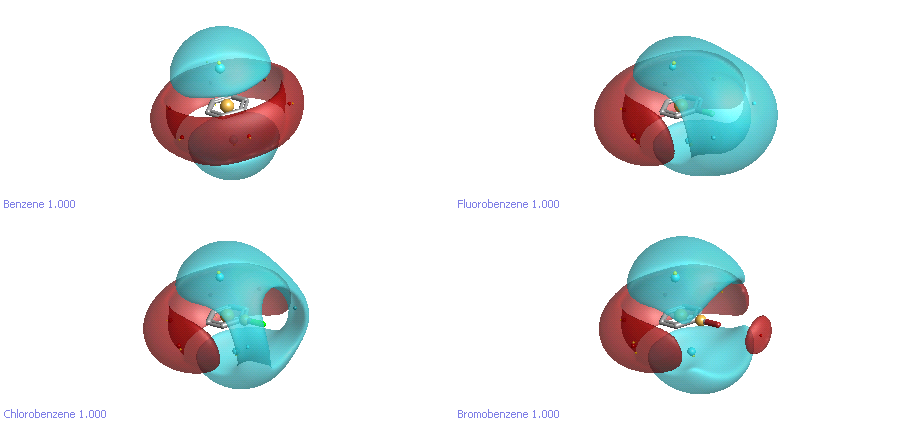
Finally, let’s take a look at the halobenzenes. The electrostatic environment around these is now widely recognised to be much more complicated than we realised. One of the goals in parameterising the new XED force field (XED FF3) was to reproduce the experimental and theoretical data about the behaviour of halogens on aromatic rings. As you can see in Figure 3, the electrostatics are complicated. Fluorine is simply delta-negative, while chlorine has a “hole” out along the bond axis where there is little or negative charge. Bromine extends this even further, to the extent that it has a small positive “hat”. These subtleties make a real difference to how the halogens behave when interacting with protein atoms.
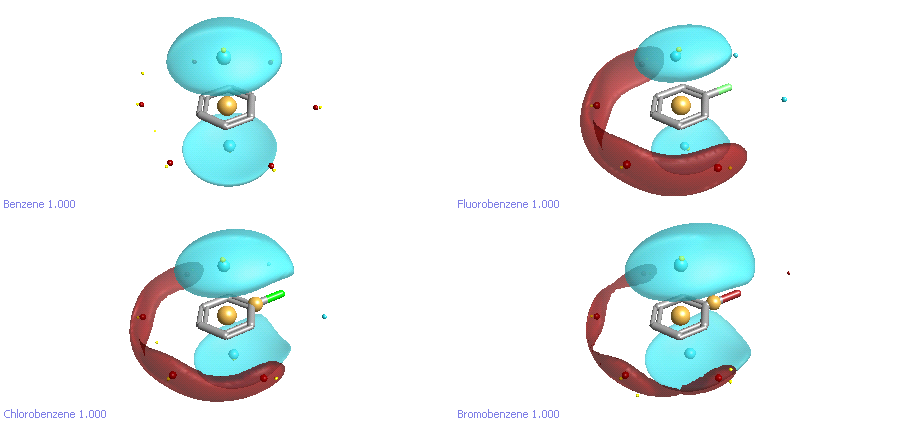
If we take another look at halobenzenes at a different electrostatic contour level, another set of differences becomes apparent (Figure 4). Benzene has the well-known pi-cloud above and below the plane of the ring, and a weak torus of positive field around the edge. Fluorobenzene, as we noted earlier, is significantly more polarized: the pi clouds are slightly weaker, and the edge of the ring away from the halogen is significantly more positive. It’s interesting to look at the trend in the size of both the pi cloud and the +ve potential at the back of the ring as we go down the halogens. Going from F to Cl to Br, the pi cloud on the ring increases in size, and the ring polarization decreases. This reflects the well-known properties of chlorine and bromine as weak deactivators for electrophilic aromatic substitution due to a combination of inductive withdrawal and resonance donation. All three halobenzenes have a smaller pi cloud than benzene.
So, in summary, Cresset’s fields are useful for much more than aligning and scoring compounds. Visual inspection of the field patterns can give you real insight into the details of how your molecule might be interacting with a protein active site, and the effects (both local and non-local!) of making changes.