Improving PROTAC properties via single-point changes to linkers
We explore how computational methods can be applied to proteolysis targeting chimera (PROTAC) design, to effectively tackle some of the ...
News
 The metabotropic glutamate receptors (mGluR) have become popular and important targets in small molecule drug discovery. In particular there is increasing interest in and clinical trials of mGluR5 antagonists in the treatment of anxiety, depression, pain, gastro-esophageal acid reflux disease (GERD), Parkinson’s disease, epilepsy, and Fragile X Syndrome (FXS). The allosteric binding site of mGluR5 , located within the transmembrane region, is generally considered to more likely to lead to effective medicines than other binding sites
The metabotropic glutamate receptors (mGluR) have become popular and important targets in small molecule drug discovery. In particular there is increasing interest in and clinical trials of mGluR5 antagonists in the treatment of anxiety, depression, pain, gastro-esophageal acid reflux disease (GERD), Parkinson’s disease, epilepsy, and Fragile X Syndrome (FXS). The allosteric binding site of mGluR5 , located within the transmembrane region, is generally considered to more likely to lead to effective medicines than other binding sites
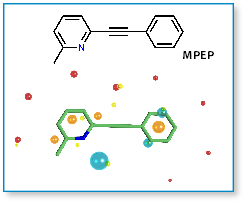
One of the prototypical small molecule mGluR5 allosteric antagonists is MPEP (2-methyl-6-(phenylethynyl)pyridine). Using MPEP as a starting point we searched for bioisosteric replacements that would introduce novel IP in this well worked area. In both cases, bioisosteres were retrieved using FieldStere.
FieldStere searches a up to 600,000 fragments for bioisosteres that exhibit similar shape and electronic properties when placed in the context of the final molecule. FieldStere uses molecular interaction Fields to represent the key binding interactions of a molecule giving a close approximation to the protein’s view of a potential ligand. Using the Field descriptors in FieldStere gives a wide range of bioisosteres, from the obvious replacements, through less obvious to completely non-obvious.
Using FieldStere on MPEP, two separate experiments were performed. Below are shown a selection of the results obtained when searching for replacements for the central alkyne (left column below) and for the pyrido-alkyne section of the molecule (right column below). In both cases unreported, novel structures were found together with a significant number of previously reported actives. The position of the found actives was irrespective of the 2D similarity of the final molecule to MPEP.