Future-proofing Cybersecurity in Drug Discovery
The pharmaceutical and biotech sectors suffer more data security breaches than any other industry, with 53% resulting from malicious activity. To protect against potential ...
News
Since its publication on J. Med. Chem in 1972, J.G. Topliss’ operational scheme for aromatic substitution,1 commonly known as ‘the Topliss tree’, has been widely used by medicinal chemists to explore the substitution pattern on a benzene ring.
This decision tree approach was designed for maximum simplicity of application by the medicinal chemists, avoiding use of computers and statistical procedures. It is based on a stepwise selection of substituents designed to progress as rapidly as possible to the most potent compounds in a series.
The Topliss tree is based on the concept, pioneered by Hansch,2,3 that the observed changes in activity following the introduction of a substituent on a benzene ring depend on its lipophilic, electronic and steric properties, which in Topliss’ original publication were represented in a quantitative manner by the physico-chemical parameters µ, σ and Es.
To complement this traditional drug design approach, we have used Torch,4 Cresset’s powerful molecular design tool for medicinal and synthetic chemists, to visualize and give new insight to the deep changes in electrostatic and hydrophobic properties introduced by the substitution patterns recommended by the Topliss tree (updated 2020):
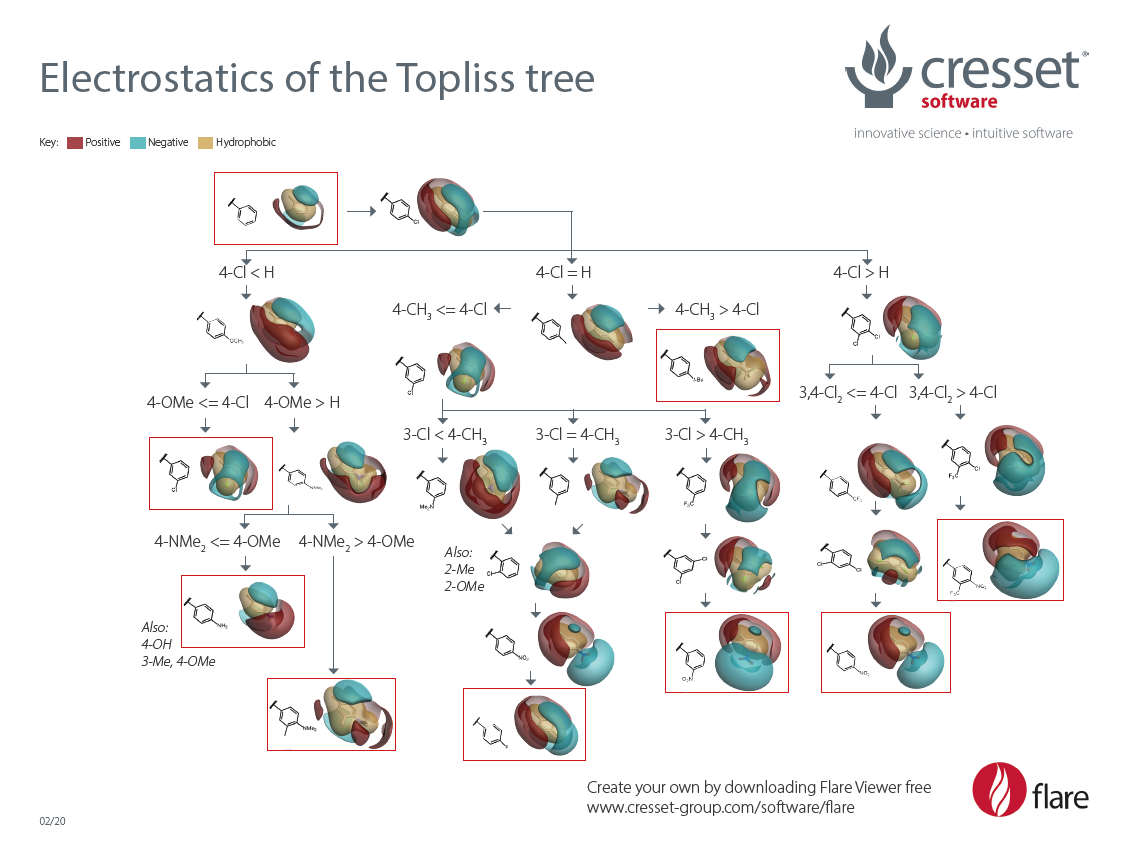
Download ‘Electrostatics of the Topliss tree’
1. J. Med Chem. 1972, 15, 1006