As kinases have become a well-established class of therapeutic targets, it’s probably fitting that the theme of the RSC kinase symposium this year was relatively conservative, although a reasonably diverse selection of kinases (table 1) and therapeutic areas (table 2) were covered. It is remarkable that so many ATP pocket blocking inhibitors (i.e., most examples described this year) have translated to clinical candidates and drugs on the market, despite the fact that kinase biology, particularly the role and mechanism of kinase modulation by multiple protein complex formation as a prerequisite for signalling, is not very well understood.
Table 1: Kinase targets 2018 symposium.
| Targets |
Number |
Kinase |
| Lipid kinases |
4 |
PI2K, PI3K, PI4K, ATM |
| Serine Threonine |
10 |
MPS1 (TTK), ERK, PKC, AMPK, CDK, LRRK2, MLK, TTBK1, PINK1, mTORC |
| Tyrosine |
3 |
ALK, EGFR, TRK |
Table 2: Kinase therapeutic areas 2018 symposium.
| Therapy |
Number |
| Oncology |
9 |
| CNS |
5 |
| Diabetes |
1 |
| Transplant rejection |
1 |
| Tropical disease |
1 |
The talk by Craig Malcolm (Promega UK Ltd) perfectly illustrated the lack of understanding of which processes involving kinases are actually operating in cells i.e., those which may, or may not, be therapeutically relevant and accessible. He described an interesting ‘different take’ on the standard kinase biochemical assay by using florescent kinase inhibitor probes as a technique to assay kinase activity in the ‘live’ cellular context.
Lipid kinases
The talks by Klaus Okkenhaug (University of Cambridge and Babraham Institute, UK), and Roger Williams (MRC Laboratory of Molecular Biology, UK), offered in depth exploration of the intricacies of the lipid kinase family and the effect of the wider molecular and cellular context of these kinases on the therapeutic outcome.
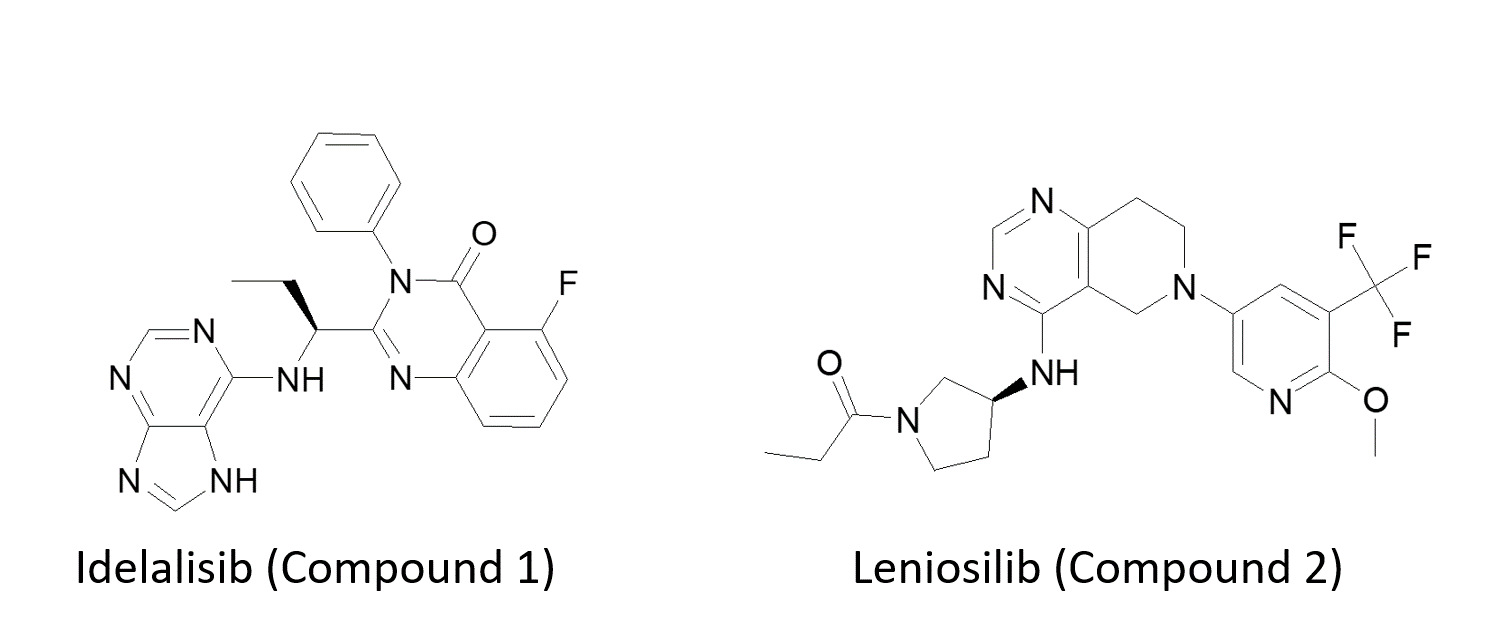
Klaus described how diverse this family is firstly with the PI3K delta inhibitor Idelalisib (1), which in combination therapy with biologic Rituximab for Chronic Lymphocytic Leukemia (CLL), serves to draw cells out of the lymph nodes so that they can be targeted by antibody treatment. The role of PI3K delta in immuno-oncology was evident in combination with CSF1 as this synergizes knocking out tumor cell protection. Finally, Leniosilib (2), is an anti-inflammatory agent through selective PI3K delta inhibition.
In his talk, Roger described the VPS34 system and how the nature of multi-protein architecture governs specific membrane interactions and thus activation of this complex assembly.
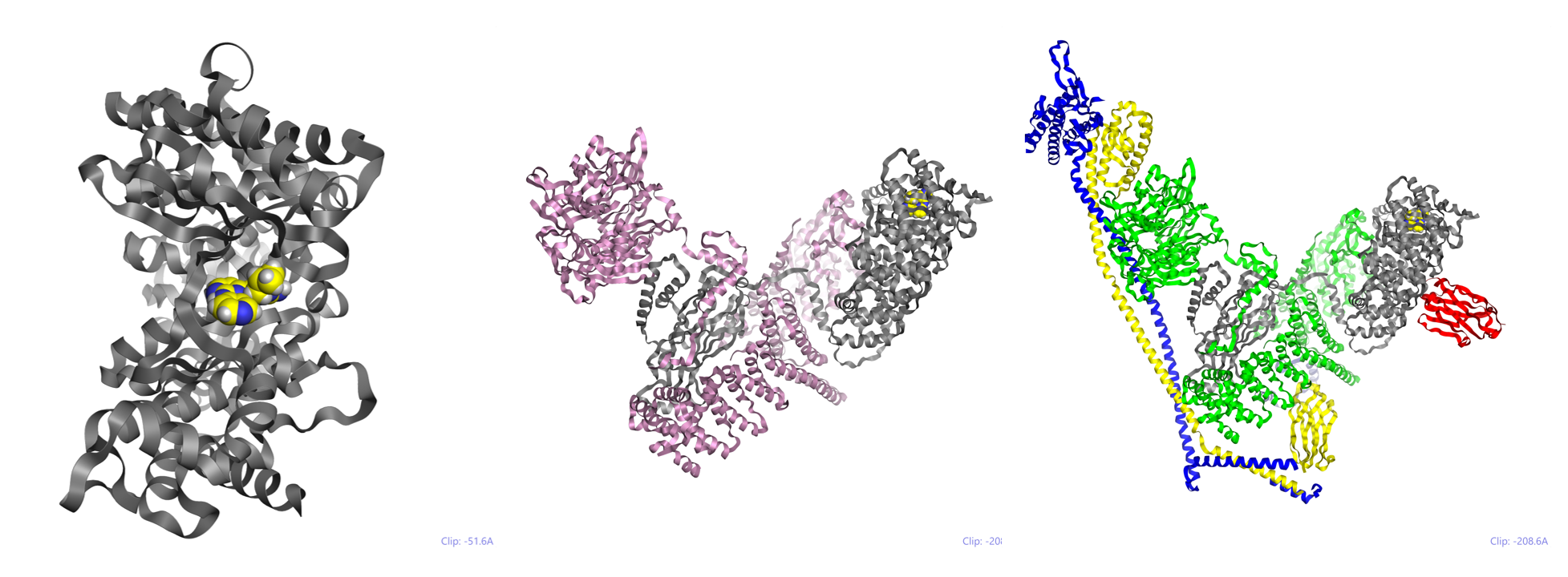
Figure 1: Left – VPS34 (human PI3K) with inhibitor (PDB code 4PH4); center – yeast VPS34 / VPS15 complex (PDB code 5KC2); right – the larger yeast complex (PDB code 5DFZ) showing the context of PI3K catalytic domain (in grey across the structures).
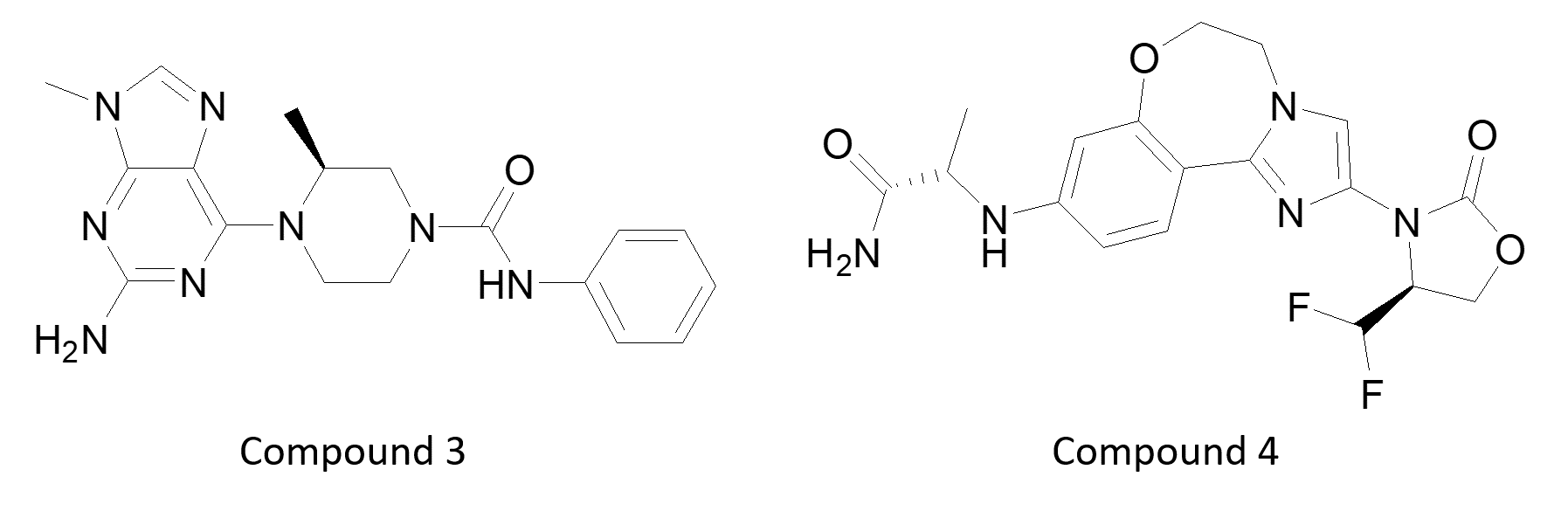
In addition, James Reuberson (UCB, UK) went on to describe a PI4K 3-beta inhibitor for immune suppression (3) and Emily Hannan talked about Genentec’s selective PI3K alpha inhibitor and degrader (4) for breast cancer. The latter molecule stimulates removal of PI3K, presumably by stabilizing a conformation that can be more readily ubiquitinated.
Kinase activators
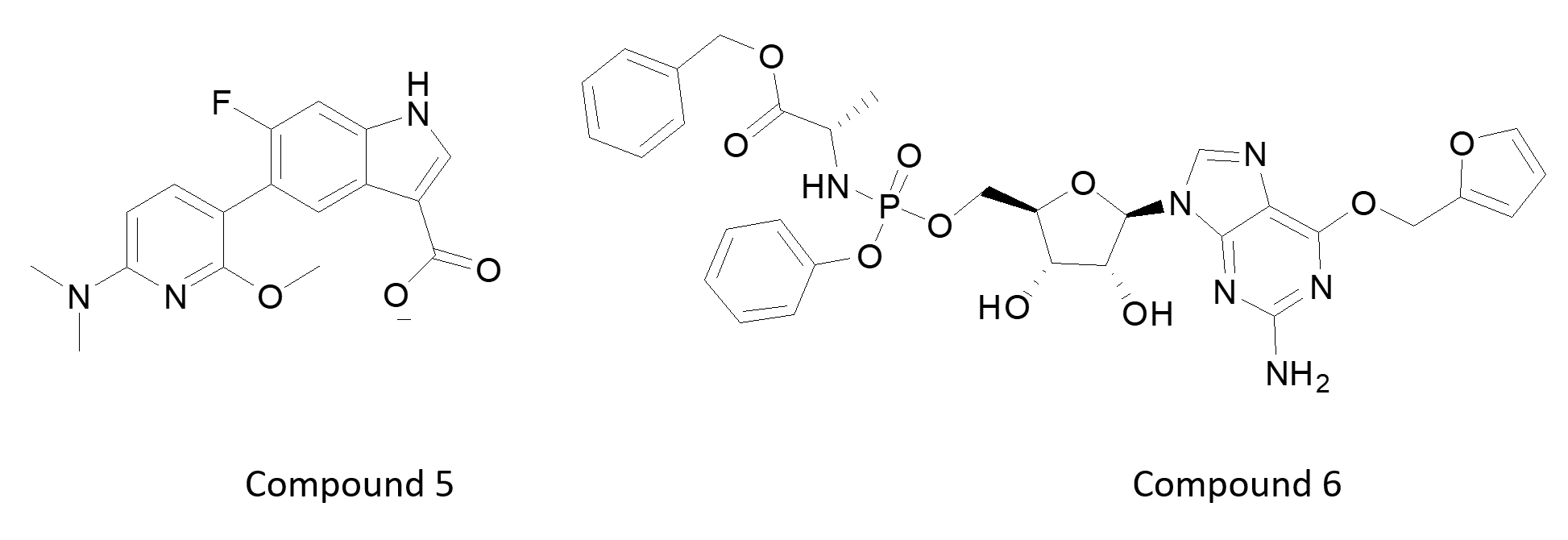
Exceptions to the general rule of ‘ATP site binding inhibition’ were two talks describing the discovery and development of kinase ‘activators’ of AMPK (5) and then PINK1 (6). AMPK is an unusual kinase with an allosteric modulatory site above the ATP binding catalytic site. PINK-1 is also an unusual kinase which has extended loops around the catalytic domain responsible for binding and phosphorylating ubiquitin. PINK-1 interacts directly with an E3-ligase at the surface of mitochondria and modulates mitochondrial autophagy. Youcef Mehellou, (Cardiff School of Pharmacy and Pharmaceutical Sciences, UK) described the use of novel ATP mimetic prodrugs which activate a form of PINK-1 that is mutated in Parkinson’s disease.
Kinase inhibitors for the CNS
Unsurprisingly, most talks focused on oncology as the major topic. Another sub-theme was accessing the CNS with kinase inhibitors for brain tumors or with CNS penetrating ligands for psychiatry or neurodegenerative diseases. Compound 6 is an example of a CNS penetrating pro-drug. Most other examples focused on optimizing inhibitors either by multiple parameter optimization of calculated properties, or using experimental measurements such as compound measurement in brain slice homogenate as a component of their KPU metric. These include: a TTBK1 inhibitor from Lundbeck (7) for Alzheimer’s, the ATM inhibitor from AZ (8) for Glioblastoma, and the pan TRK inhibitor from Chugai (9) for brain metastasis. Note the absence of the classic donor-acceptor-donor hinge interaction motif.
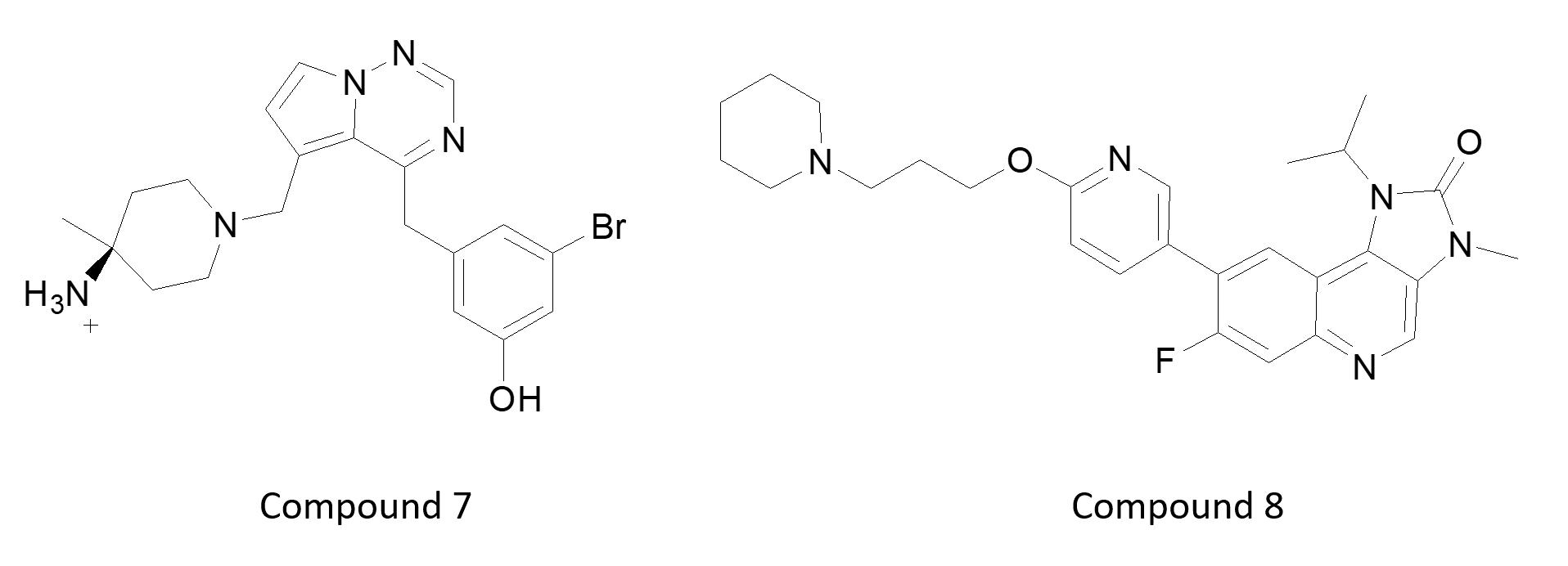
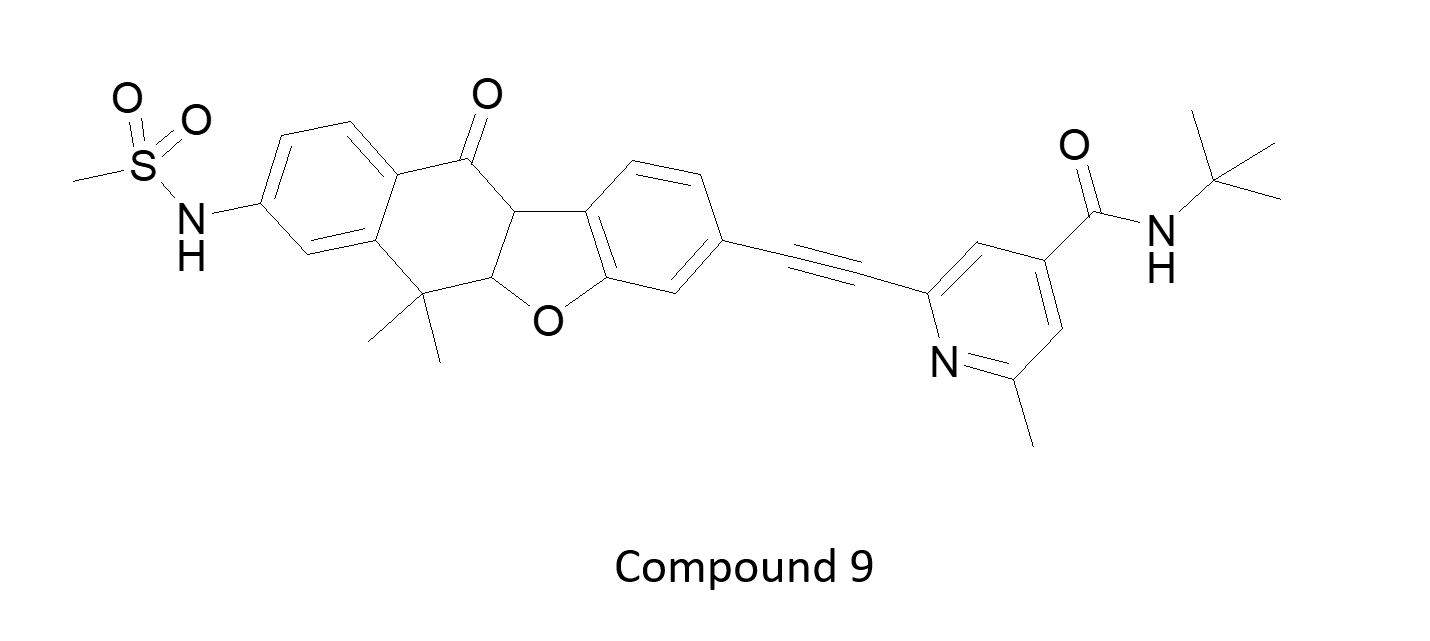
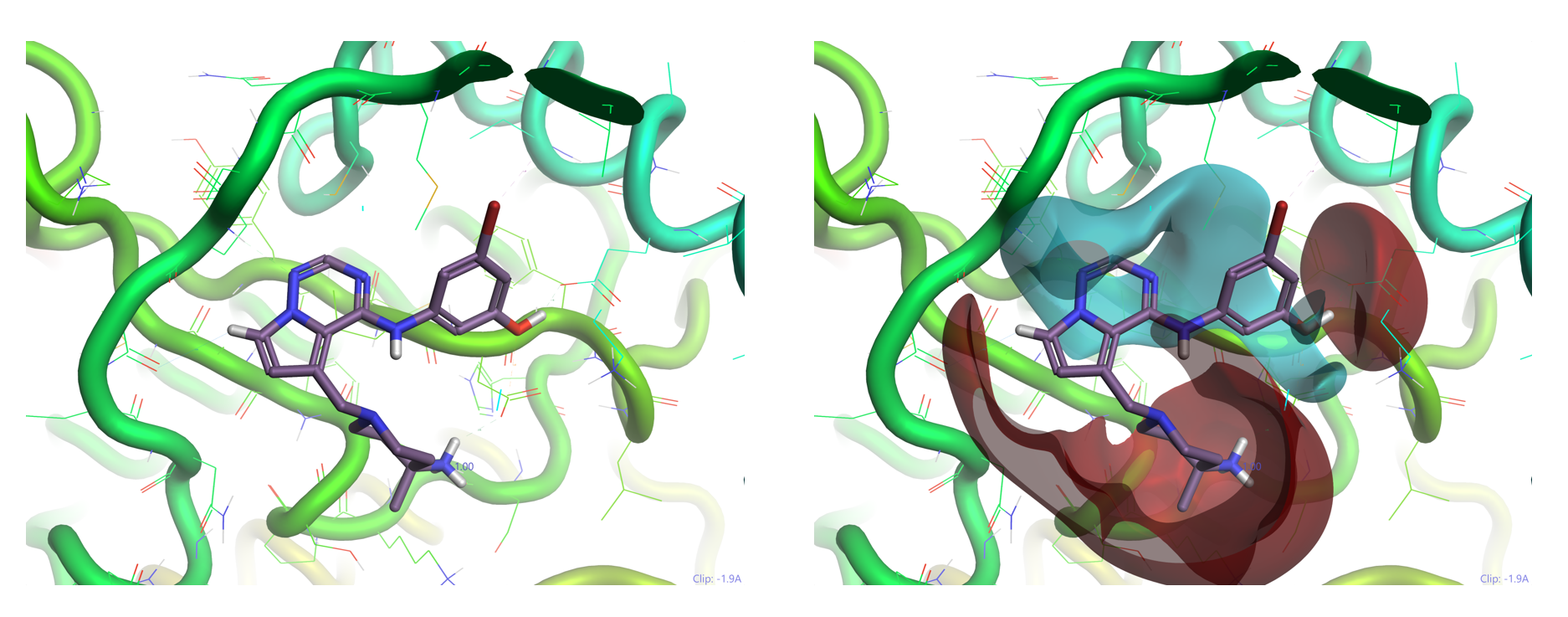
Figure 2: Lundbeck TTBK1 inhibitor (7) for Alzheimer’s disease (left – PDB code 4NFN) shows non-classical hinge interactions which are more evident using Cresset electrostatic field visualization (right) in Flare (cyan: negative potential; red: positive potential).
Conclusion
Overall the symposium was interesting and informative.
Contact us for a free confidential discussion to see how Cresset Discovery Services experts can advance your project.






