Cresset Discovery Services has extensive experience working on established and novel anti-microbial targets. We understand the unique challenges of antibiotic development and bacterial resistance and can partner with your development team to reduce your development costs and decrease time to market for new antibiotics.
Bacteria are among the most prolific and successful organisms on the face of the earth. They are essential for life and for the most part we live in harmony. However, the wrong bacteria in the wrong place can lead to serious problems, which is when we turn to antibiotics for help.
The media is predicting an antibiotic resistance apocalypse, talking up superbugs, MRSA and the failure to develop new antibiotics. Behind these headlines there is a growing need for new and novel antibiotic drugs.
Developing new antibiotic drugs has always been difficult. Many of the easier targets have been successfully used, but resistant mechanisms are constantly evolving, limiting the efficacy of current antibiotics. Most of the larger pharmaceutical companies have moved out of the area, so the development of new antibiotics has fallen to small/medium biotechs, new start-up companies, academic researchers and public private partnerships, who are all trying to fill this crucial development void. Any help in identifying and prioritizing where chemical and biological effort should be focused will only to reduce development costs but also minimize the time required to develop these much need antibiotics.
The challenges for antibiotic drug design are comparable to those for traditional drug discovery, plus the additional obstacle of the established resistance mechanisms of bacteria:
- The first and physically obstructive problem is getting antibiotics through the bacterial cell wall. This is especially true for gram negative bacteria which have an additional lipid membrane.
- Once inside the cells there is an array of efflux pumps waiting to transport the compound back out of the cell before it can have an effect.
- Bacteria have also developed enzymes specifically designed to stop antibiotics working. The best example of this is the b-lactamase proteins that decarboxylate b-lactam rings rendering penicillin and related antibiotics ineffective (figure 1).
- The final trick up the bacterial sleeve is mutagenesis. Since bacteria grow rapidly there are multiple strains of the same bacteria in a single infection event. If just one of these strains is viable and infers resistance to the prescribed antibiotic then an antibiotic resistant infection is promoted through the whole infection.
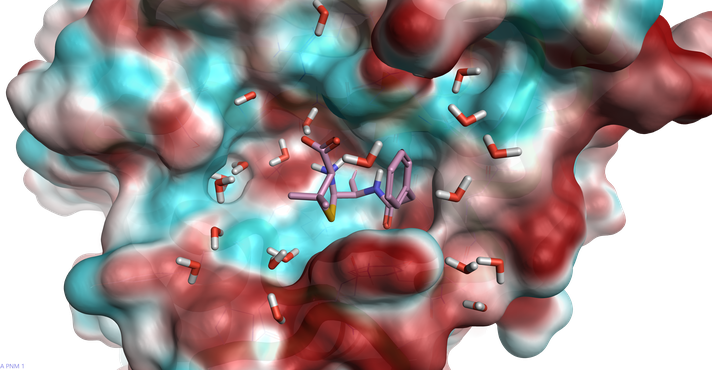
![]()
Figure 1: The electrostatic protein surface of staphylococcus aureus beta-lactamase with a decarboxylated beta-lactamase from the 1GHP Crystal Structure1 highlighting a resistance mechanism for this class of antibiotics.
How can in silico methods help to address this growing need?
Through our extensive experience working on anti-microbial targets, both novel and currently established, we understand the challenges that this class of targets offer. For example, how simply designing Lipinski type molecules that fit into an active site without the consideration of the additional bacterial resistant mechanisms is unlikely to yield favorable results.
Computational techniques such as 3D electrostatic field comparisons (figure 2) and multi-parameter optimization are key in this field where cell penetration, efflux rates, frequency of mutation are factors. Potentially, other project-specific properties must also be considered in addition to normal drug properties.
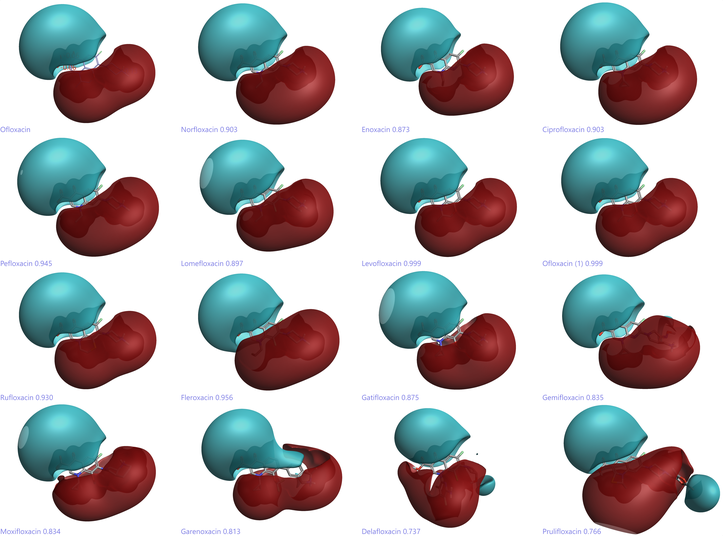
Figure 2: Field comparison of a set of fluoroquinolones antibiotic compounds displaying the conserved electrostatic nature of this class of compounds which is key for DNA intercalation and complex stability.
These advanced techniques in addition to traditional methods provide customers with the rationalization underpinning the predictions. This means that Cresset Discovery Services adds value to your project long after our direct engagement has finished. In addition, Cresset’s exceptional visual analysis tools help to understand and share ideas.
We collaborate with your development team to leverage experience to identify the key proteins to investigate, identify the best hypothesis to test, and ultimately to choose the most promising compounds for synthesis, accelerating your antibacterial project towards successful completion.
Advance your antibiotic project
Contact us for a free confidential discussion to see how we can advance your antibiotic project.
Reference
- Structures of the Acyl−Enzyme Complexes of the Staphylococcus aureus β-Lactamase Mutant Glu166Asp:Asn170Gln with Benzylpenicillin and Cephaloridine Celia C. H. Chen and Osnat Herzberg Biochemistry, 2001, 40 (8), pp 2351–2358

