We’ve been writing an article for Current Computer-Aided Drug Design that describes many of the features of our software. As part of this we came across a nice example of the advantages of assessing bioisosteric replacements in product space rather than in fragment space. Consider the replacement of the cyclic lactone group of rofecoxib with either a thiazolotriazine or benzimidazole below.
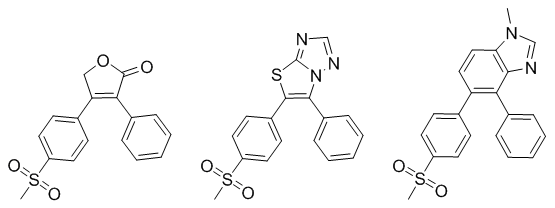
Both the replacements have two rings in place of the original one and both contain an H-bond acceptor. On the face of it these look quite similar. However, adding fields to these three molecules gives a remarkably different view:
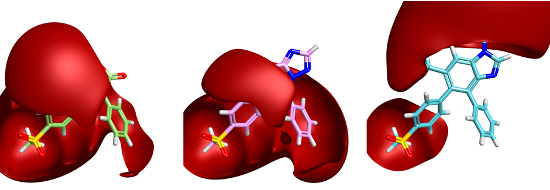 The positive field is shown contoured at 1.5kcal/mol and clearly shows a significant difference in the field surrounding the pendant phenyl group. This visual field difference is mirrored in our field score. Using FieldStere to search for replacements and asking for all aromatic ring fragments to be considered give the thiazolotriazone as the 9th result. The benzimidazole is at position 1,432 in the FieldStere list of 1,470 possible replacements. Intriguingly, as far as I can tell the benzimidazole is unreported as a COX-2 inhibitor whilst the thiazolotriazine compound shown is a 10nM active.
The positive field is shown contoured at 1.5kcal/mol and clearly shows a significant difference in the field surrounding the pendant phenyl group. This visual field difference is mirrored in our field score. Using FieldStere to search for replacements and asking for all aromatic ring fragments to be considered give the thiazolotriazone as the 9th result. The benzimidazole is at position 1,432 in the FieldStere list of 1,470 possible replacements. Intriguingly, as far as I can tell the benzimidazole is unreported as a COX-2 inhibitor whilst the thiazolotriazine compound shown is a 10nM active.

