Future-proofing Cybersecurity in Drug Discovery
The pharmaceutical and biotech sectors suffer more data security breaches than any other industry, with 53% resulting from malicious activity. To protect against potential ...
News
“Little Jack Horner
, sat in the corner, eating a Christmas pie;
He put in his thumb, and pulled out a plum, and said ‘What a good boy am I!”
Traditional English
As you’re reading this, you may be wondering about whether the Cresset team might have been into the eggnog again. What do coal and Christmas pie have to do with computational chemistry?
Aromatic compounds, like those produced during incomplete coal combustion, can be complex in how they interact with one another. Key to those non-covalent intermolecular interactions are π-electrons (get it? Pie?); and these types of non-covalent interactions are extremely common in protein-ligand interactions and self-assembled systems.
Despite the common occurrence of π-cloud electrostatic interactions, traditional atom-centered force fields (with atom-centered charges) are incomplete in their description of these types of dichotomous (simultaneously both electrostatic and hydrophobic) interactions. And while traditional approaches will get you some of the way to truth, it misses induction and other through-bond / through-space interactions that result from the electronic anisotropy.
π-π interactions take on a number of motifs (edge-to-face, offset parallel stacking, etc.) in both small-molecule crystals and protein-ligand crystal structures. Traditional MM force field calculations predict these interactions to be face-to-face. To replicate what is seen experimentally (e.g., edge-to-face), an electronic structure method (i.e., QM) or an “electron-aware” force field would be required.
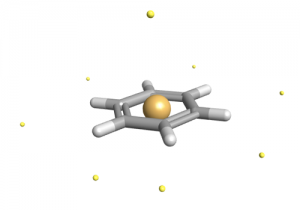
Underlying all of Cresset’s software is the XED (eXtended Electron Distribution) force field. XED is now in its 3rd iteration, and has been a 30-year work-in-progress for Cresset’s founder, Andy Vinter.
The XED approach is unique in that it explicitly represents electron anisotropy by using a distribution of charged “orbital points” around the molecule (depending on atom hybridizations), effectively moving the negative charge OFF the nucleus. Each XED point can thus be seen as p-orbital electrons (lone pairs, or Π-cloud delocalized pairs; and these points are included in the MM optimization.
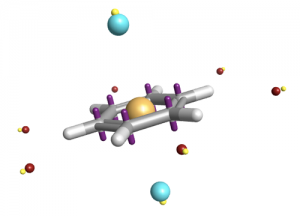
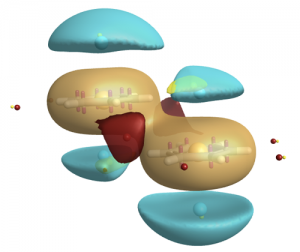
XED, with its off-nucleus charges, avoids the pitfalls of the electrostatic potential fitting approach. The XED charges are set using an empirical charge generator that takes into consideration both electronegativity and electron drift. When you think of what atoms are really like, it makes sense that the positive charge is on the nucleus, and the negative charge is not!
While enjoying a nice glass of eggnog, I encourage you to take a peek at the following papers:
Extended electron distributions applied to the molecular mechanics of intermolecular interactions
Vinter. J., CompAided Mol Des., 1994, 8, 653-668.
An Evaluation of Force-Field Treatments of Aromatic Interactions
Chessari et al., Chem. Eur. J., 2002, 8 (13), 2860-2867
Happy Holidays!

Rae Lawrence
Account Manager, North America (West)