Improving PROTAC properties via single-point changes to linkers
We explore how computational methods can be applied to proteolysis targeting chimera (PROTAC) design, to effectively tackle some of the ...
News
The new release of Spark V10.3, Cresset’s chemistry software for bioisosteric replacement, brings new fragment databases and new advanced filters to control the chemical and physico-chemical space on results. In this case study we apply these new features to the search for new terminal groups for a series of Dipeptidyl Peptidase IV (DPP-IV) inhibitors.
We downloaded the pdb file 1X70, loaded this into Spark V10.3, split the file into ligand and protein excluded volume (keeping Chain A only) using the new protein import facility. We selected the head group of the ligand for replacement then chose to search the Chembl_common, VeryCommon, Common, LessCommon and Rare databases.
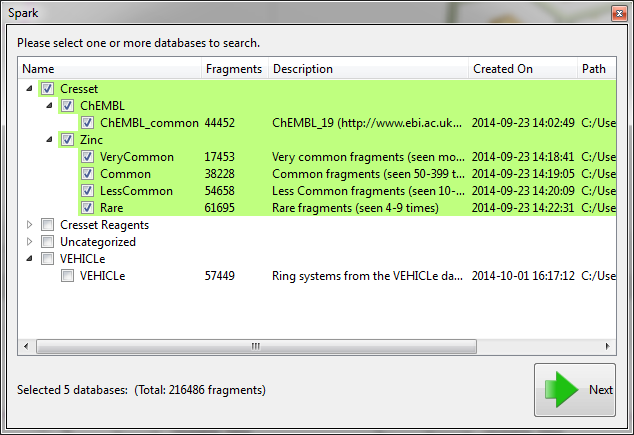
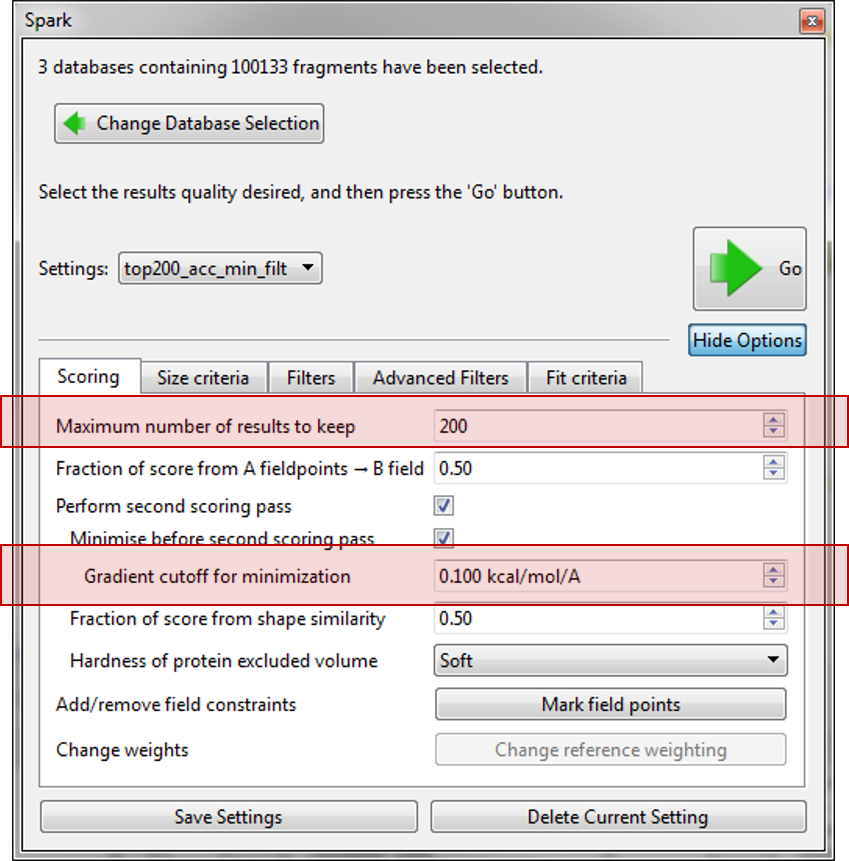
Before starting the the ‘Accurate but slow’ experiment we altered some of the parameters which have been introduced in this version. First on the ‘Scoring’ tab we limited the number of results to just 200 and improved the minimization of the final molecules by decreasing the gradient cutoff during the minimization to 0.1 kcal/mol/A. Next, on the ‘Size criteria’ tab we reduced the maximum number of rotatable bonds permitted in a fragment to 3.
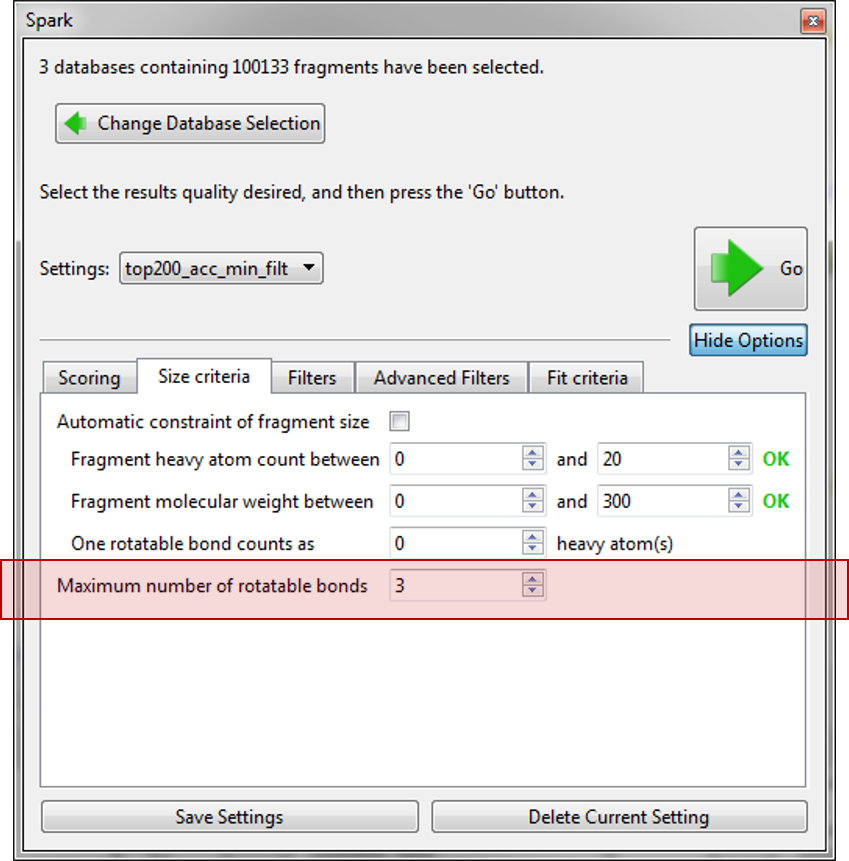
Finally, we used the new ‘Advanced Filters’ tab to control the physical properties of the final molecules so that we only have molecules with a calculated logP within 1 and 3.5 and a TPSA between 40 and 90. As we expect to use these settings in the future they were saved to a new name ‘top200_acc_min_filt’.
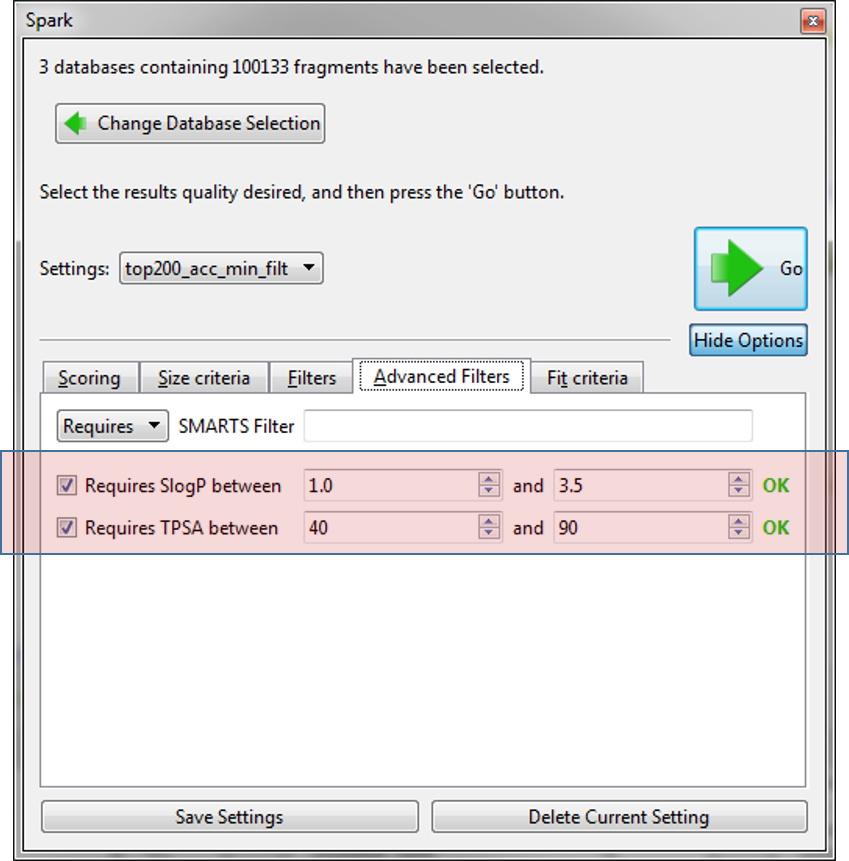
The calculation was performed using Cresset Engine Broker to distribute the calculation from the host (Windows® 7 based) laptop to to Cresset’s Linux® farm. Running on an additional 20 CPUs the calculation took less than 30 minutes to complete.
We used the new tile view to summarize each result. It was configured with the radial plot (setup with Score, MW, logP, TPSA and 2D similarity) for each compound together with selected textual data (Rank, Favorite, Database source, and Unstable flag). The first 30 results are shown in the screen shot below.
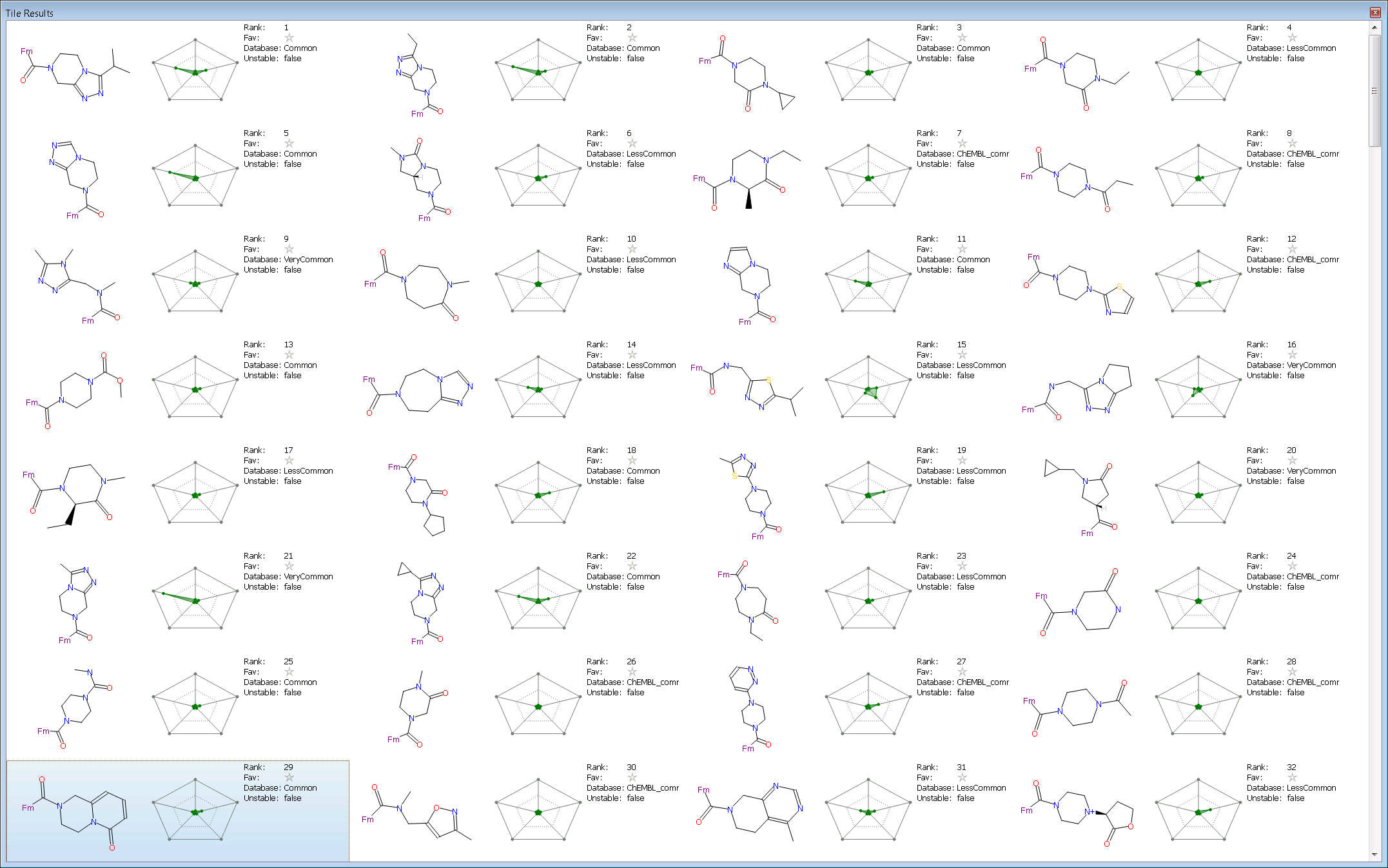
The first two results are members of the same series as the query (one of which is a known nM inhibitor) which is a nice validation that that the experiment was setup reasonably. These are followed by a couple of 3-oxo-piperazine which is also a known active scaffold for DPP-IV. Interestingly Spark suggests a 3-oxo-tetrahydro-imidazopyrazine at rank 6 which is quite different to the starter molecule and has excellent properties (small shaded area in the radial plot). This is also a known to be active at DPP-IV with the compound shown having a reported pIC50 of 5.9. At rank 9 in the results is a ring opened version of the starter molecule and then at rank 10 a 1,4-diazepanone which is again a known scaffold for DPP-IV. The next few results are similar to previous results or are known DPP-IV scaffolds such as the N-aromatic acyl piperazines.
Looking down the results for a scaffold not already known to be active at DPP IV gives the 3,4-dihydro-1H-pyrido[1,2-a]pyrazin-6-one at rank 29. This looks an interesting replacement with good properties and room for further substitution. The fragment was found from the ‘Common’ database and appears readily available.
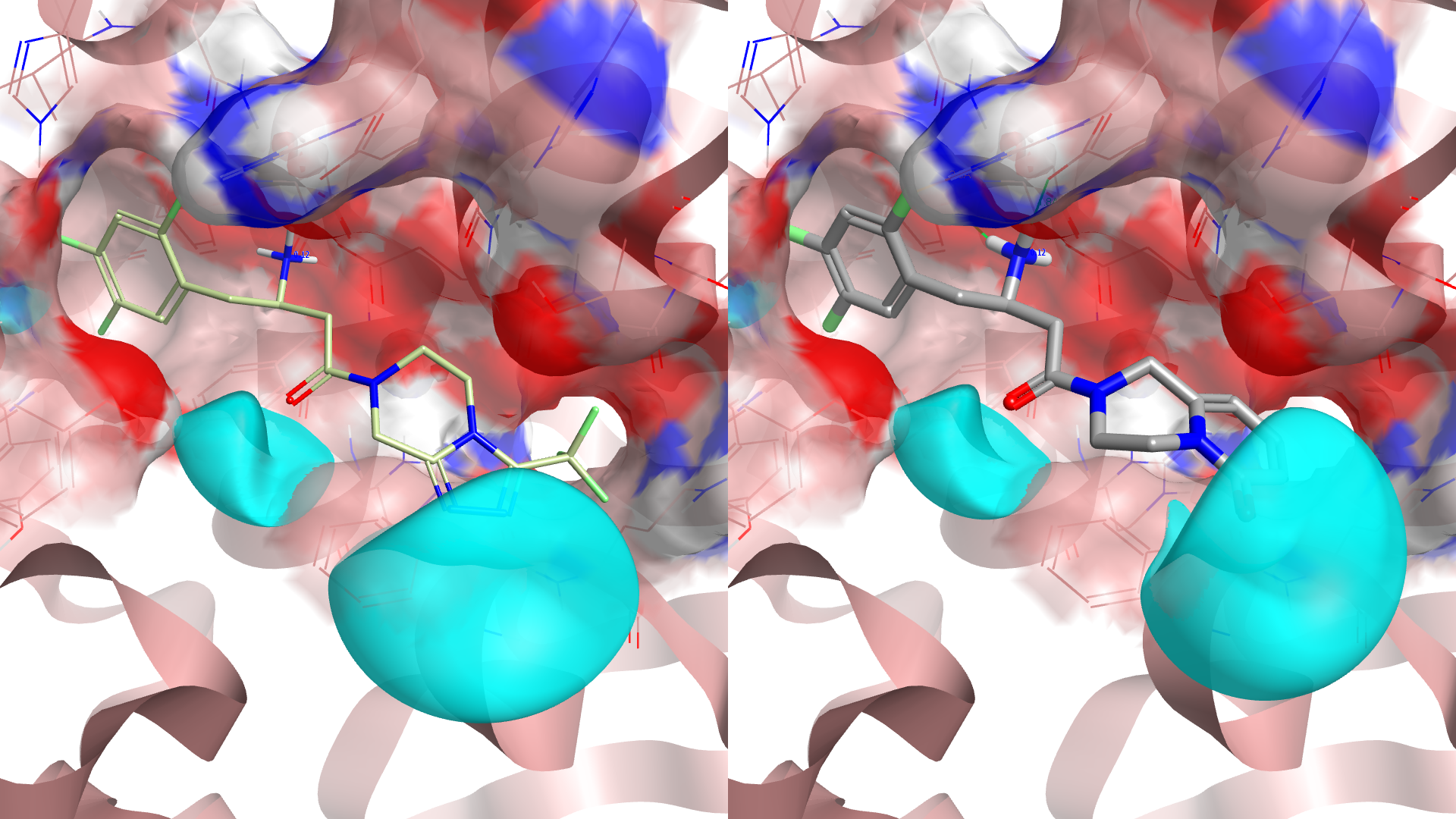
These results demonstrate the power of Spark: a simple 30 minute experiment located not only numerous known scaffold replacements, giving confidence that Spark is indeed producing compounds with the desired properties for activity but also structurally-novel suggestions in clear IP space. The new tile view allows the results to be rapidly triaged by eye, allowing you to focus in on the most desirable replacements quickly and easily.
Contact us to find out more.